Abstract
Introduction
Acute Promyelocytic Leukemia (APL) is a subtype of AML characterized by pancytopenia, and bleeding. The cause of bleeding is due to thrombocytopenia and disseminated intravascular coagulation. Excellent targeted drugs are available with cure rates >90% in clinical trials treated on a protocol. Given the nature of APL, ≈30% of patients in the general population die during induction, due to bleeding, differentiation syndrome (DS) and infection.
Immediate transfusion support, ATRA initiation, and prevention of DS are key to decreasing early deaths. In the leukemia community, concerns exist about prompt availability of ATRA, blood bank support, treatment protocols, and trained physicians in community hospitals. We conducted a survey to collect data on these issues required for successful treatment of APL.
Methods
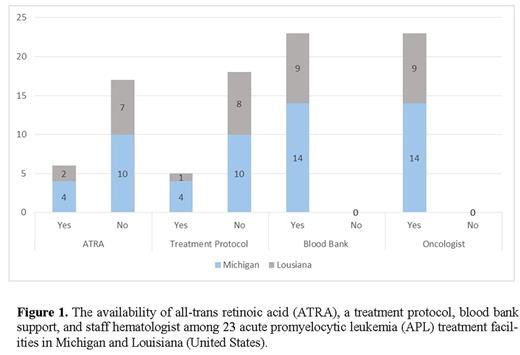
Using Surveillance, Epidemiology, and End Results (SEER) registry data, we identified the states of Michigan (MI) (population: 9.9 million) and Louisiana (LA) (population: 4.7 million) due to their low one year APL survival rate. All eligible hospitals (253) were obtained from the Data Medicare online directory (https://data.medicare.gov/). Each hospital was contacted to exclude non-APL treatment centers. We contacted the remaining 23 APL treatment centers (9 LA; 14 MI) and conducted a telephone survey of 7 questions (Table 1) with a pharmacy staff member, other hospital staff and hematologists.
Results
ATRA was available (on formulary and/or in stock) at 6 of the 23 hospitals. 4 of the 6 hospitals with ATRA were affiliated with a school of medicine (SOM).
7 hospitals answered a follow-up question about the lack of ATRA. Common answers were the pharmacist was unfamiliar with the drug (71%) or there had not been a recent request (29%).
For 17 hospitals that did not have ATRA, responses when asked about the viability of obtaining it, were:
Needs approval of P&T Committee, 7/17
Could be available within one to two days, 3/17
Could be available within two weeks, 3/17
Could not give an exact timeline, 4/17
Regarding availability of a written protocol, 5 of 23 hospitals stated they follow a protocol for treating APL. Of the 5 hospitals, only 1 was not affiliated with a SOM. Furthermore, of the 5 hospitals with a protocol, 3 (2 SOMs) have their own written protocol and 2 (1 SOM) follow the National Comprehensive Cancer Network (NCCN) guidelines. For the 18 hospitals that lacked a protocol nor followed the NCCN guidelines, an expedited and tailored approach was used based on patient's age, blood counts, and health issues.
All APL treating hospitals had staff hematologists and a blood bank with the capability to meet the transfusion requirements.
Conclusion
Despite the amount of literature about early deaths and early ATRA introduction to treat APL, many hospitals are unable to meet this demand due to lack of prompt availability of the drug. All APL treating hospitals had trained physicians and adequate blood bank support. Since most of the hospitals surveyed do not have a treatment protocol, we believe the outcome could be improved if a simplified set of treatment guidelines are developed for all hospitals treating APL.
Arellano: Cephalon Oncology: Research Funding. Kota: Takeda Pharmaceuticals: Consultancy; Leukemia Lymphoma Society: Research Funding; Incyte: Consultancy; Xcenda: Consultancy; Pfizer: Consultancy; Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal